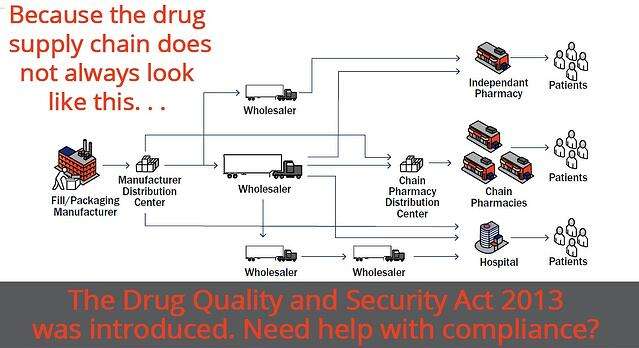
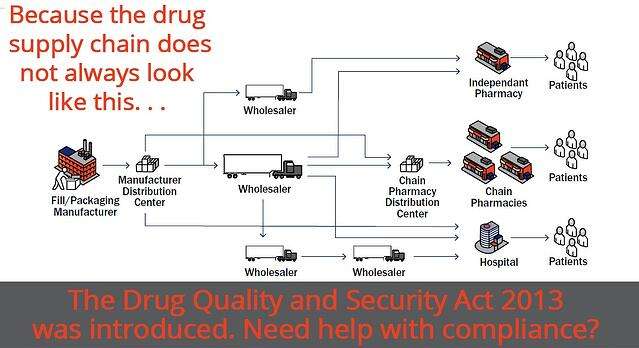
The drug supply chain security act establishes requirements to facilitate the tracing of prescription drug products through the pharmaceutical supply distribution chain with the primary goal of this bill being to protect patients from comprised and unsafe medicines.
Drug Supply Chain Security Act Details
The bill's multi-faceted steps include:
- Establishing standards for the exchange of transaction documentation for drug manufacturers, wholesalers, dispensers, re-packagers, and third party logistics providers which shall include documentation of transaction information, transaction history, and transaction statements at each transfer of ownership including returns.
- Ensuring that drug manufacturers, wholesalers, dispensers, and re-packagers use only authorized trading partners.
- Investigating suspect products; and handling illegitimate products, including quarantining, disposing, notifying authorities and trading partners if a product is unsanctioned.
The changes required by the act so far have been relatively easy to follow without need for outside assistance; the act requires that a manufacturer needs to:
- Deal with authorizing trading partners only
- Provide transaction information to trading partners
- Quarantine and investigate suspect products
- Identify and remove illegitimate products, and notify FDA and trading partners
- Trace drugs by lot number.
As we want to aid our manufacturers and those in the life sciences, we are providing a time line below for manufacturer's and re-packagers of what the drug supply chain security act requires over the next ten years:
The next steps that need to be in place before November 27, 2017, will need strong manufacturing software like Dynamics AX as the law will require:
- the placement of of a unique serial number on each package of drug
- requires the establishment of technologies to permit verification of those serial numbers to ensure the drug's legitimacy
- provide transaction information to trading partners in electronic format: what drugs were shipped, when, and to whom. Not only that but in some cases for controlled substances, the law is more detailed even down to the gram of what ingredient was used by whom, and when.
By 2023:
Product tracing will be required to help in the event of a recall or in the identification of a suspect product.
By 2027:
An electronic trace ability system will be required down to the the unit level, meaning even the smallest individual package of a drug that is sold by a manufacturer, needs to be traced.
This act requires a modern IT system and a unified, integrated enterprise resource planning (ERP) solution that provides full control over operational, manufacturing, and logistics processes. Below find the major requirements of the bill and how Dynamics AX manages them flawlessly.
1. Product Tracing & Tracking With Hand Held Devices
Our experience with companies that deploy the AX – which leverages Dynamics AX, SQL Server, SharePoint and Office – is that Dynamics AX can meet your pharmaceutical manufacturing, documentation and tracing needs. Dynamics AX, developed with proactive execution in mind, supports end-to-end transparency, making possible ingredient/product tracking across the supply chain, back and forth from a particular time/event. The solution can also be combined with industry-specific systems to natively support many ERP and quality-related workflows as well as trace raw materials with hand held devices. In addition, the system can be set to compare ingredient/product characteristics against FDA’s standards, allowing pharmaceutical companies to detect, address, correct and prevent importing issues.
2. Alerts Made Systemically If Corrective Action Plan Needed
Dynamics AX not only provides in-depth insight into business processes to help organizations operate with greater agility, but also enables users to create corrective action plans and alerts that are automated for various ‘what-if’ situations in order to divert dangerous goods or a missed step in the chain of custody.
3. Control of pharmaceutical ingredients/end products until reaching the end user.
Dynamics AX delivers a reliable drug supply chain security act solution for managing turnaround and delivery times when dealing with non-confirming products; for linking diagnostic information to appropriate corrective measures; and for tracking problems by type (e.g. long-term, short-term, urgent, etc.). With this solution, pharmaceutical groups will be able to ensure quality within supply chains until products reach the end user. Another benefit to AX is that it allows compliance, and language translation at the same time, making it easier for your company to do business internationally.
Clients First is committed to making the complexities of the drug supply chain security act time-line manageable through implementing ERP and better business processes. We operate both nationally and internationally, our AX consultants headquartered in Minneapolis/St. Paul, Minnesota can be reached at 877.428.7205, and our Dallas/Fort Worth, Texas office can be reached at 800.331.8382. Or email us by clicking on the links above.
Sources:
Tags: Microsoft Dynamics AX, Cloud ERP SaaS, Dynamics 365 for Operations
 Need Help With Drug Supply Chain Security Act Compliance?">
Need Help With Drug Supply Chain Security Act Compliance?">